Treatments & Services
Advanced Cancer Care, Every Step of the Way
Get the latest treatment options, including chemotherapy, radiation, immunotherapy, hormone therapy, and biologic therapy—plus integrative support that cares for the whole person.

Medical Oncology
Medical oncology focuses on diagnosing, treating, and managing cancer using medications that work throughout the body. Medical oncologists use a wide range of therapies, including chemotherapy, immunotherapy, targeted therapy, hormone therapy, and other systemic treatments. They coordinate closely with surgeons, radiation oncologists, and many other specialists to ensure each patient receives well-rounded, personalized care.Patients may be referred to a medical oncologist after a concerning test result, a biopsy, or a diagnosis made by another specialist. Because every patient and every cancer is different, medical oncologists develop individualized treatment plans based on the type of cancer, its stage, and each patient’s overall health and goals. Throughout treatment, they monitor progress, adjust therapies as needed, and support patients through symptoms, side effects, and the emotional challenges of a cancer diagnosis.

Hematology
Hematology focuses on diagnosing and treating conditions that affect the blood, bone marrow, and lymphatic system. Hematologists care for patients with both benign (non-cancerous) blood disorders and blood cancers, helping identify the cause of symptoms such as anemia, bleeding issues, clotting problems, or abnormal blood counts. They use specialized testing to understand how the blood is functioning and develop personalized treatment plans that may include medications, infusions, transfusions, or other targeted therapies.

Surgical Oncology
Surgical oncology focuses on the use of surgery to diagnose, treat, or help prevent cancer. Surgery may be used to remove a tumor, evaluate whether cancer has spread, relieve symptoms, or support other treatments such as chemotherapy, radiation therapy, immunotherapy, or targeted therapy. The approach your surgeon recommends depends on the type of cancer, the stage of disease, and your overall health and treatment goals. Many cancer surgeries today are performed using minimally invasive techniques that can shorten recovery time and reduce discomfort. Your surgical and oncology teams work closely together to create a coordinated, personalized plan that supports both safety and long-term outcomes.
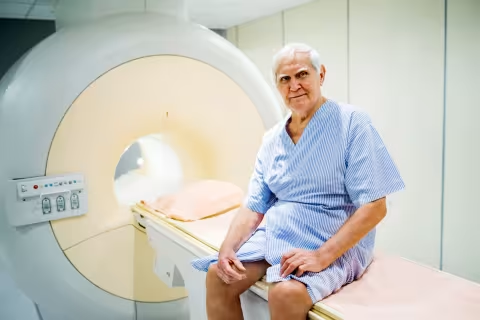
Radiation Therapy
Radiation therapy uses highly targeted, high-energy radiation to destroy cancer cells and shrink tumors. It is one of the most common treatments for cancer. Radiation may be used on its own or combined with other treatments such as surgery, chemotherapy, immunotherapy, or targeted therapy, depending on each patient’s diagnosis and care plan.Radiation therapy affects only the area of the body being treated. It works by damaging cancer cells so they can no longer grow and divide. Healthy cells in the surrounding area may also be exposed to radiation, but they can usually repair themselves and return to normal function. Your care team will plan each treatment carefully to deliver the most precise dose while protecting as much healthy tissue as possible.

Clinical Trials & Research
Clinical trials and research programs offer patients access to innovative cancer treatments that are being studied for safety and effectiveness. By participating in a clinical trial, patients may receive promising therapies earlier while helping researchers learn more about how to prevent, detect, and treat cancer. Providing clinical trial opportunities reflects a commitment to high-quality, evidence-based care, supported by a team trained to follow rigorous safety and monitoring standards. Clinical trials allow patients to explore new treatment options close to home, with the support of a dedicated oncology and research team guiding them every step of the way.

Patient Support Services
At The Center for Cancer and Blood Disorders, patient support is an essential part of our care. We offer a wide range of services designed to support the physical, emotional, spiritual, and practical needs of patients and their loved ones. These programs help manage symptoms, reduce stress, improve quality of life, and provide guidance throughout diagnosis, treatment, survivorship, and beyond. All support services are available by appointment, and many are provided at no cost to established patients.

Careity Partnership
Massage, Acupuncture, Psychotherapy, Social Workers, Chaplain, Nurse Navigation and Registered Dietitian services are provided through a partnership of the Center and Careity . Visit https://www.careity.org/ for more information about Careity.